For transparency, we require corresponding authors to provide details of their co-author contributions to the manuscript using the relevant CRediT roles. The CRediT taxonomy comprises 14 distinct roles that describe each contributor’s specific contribution to the scholarly output. The roles are: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Software; Supervision; Validation; Visualization; Roles/Writing - original draft; and Writing - review & editing. Note that not all roles may apply to every manuscript, and authors may have contributed through multiple roles. More details and an example.
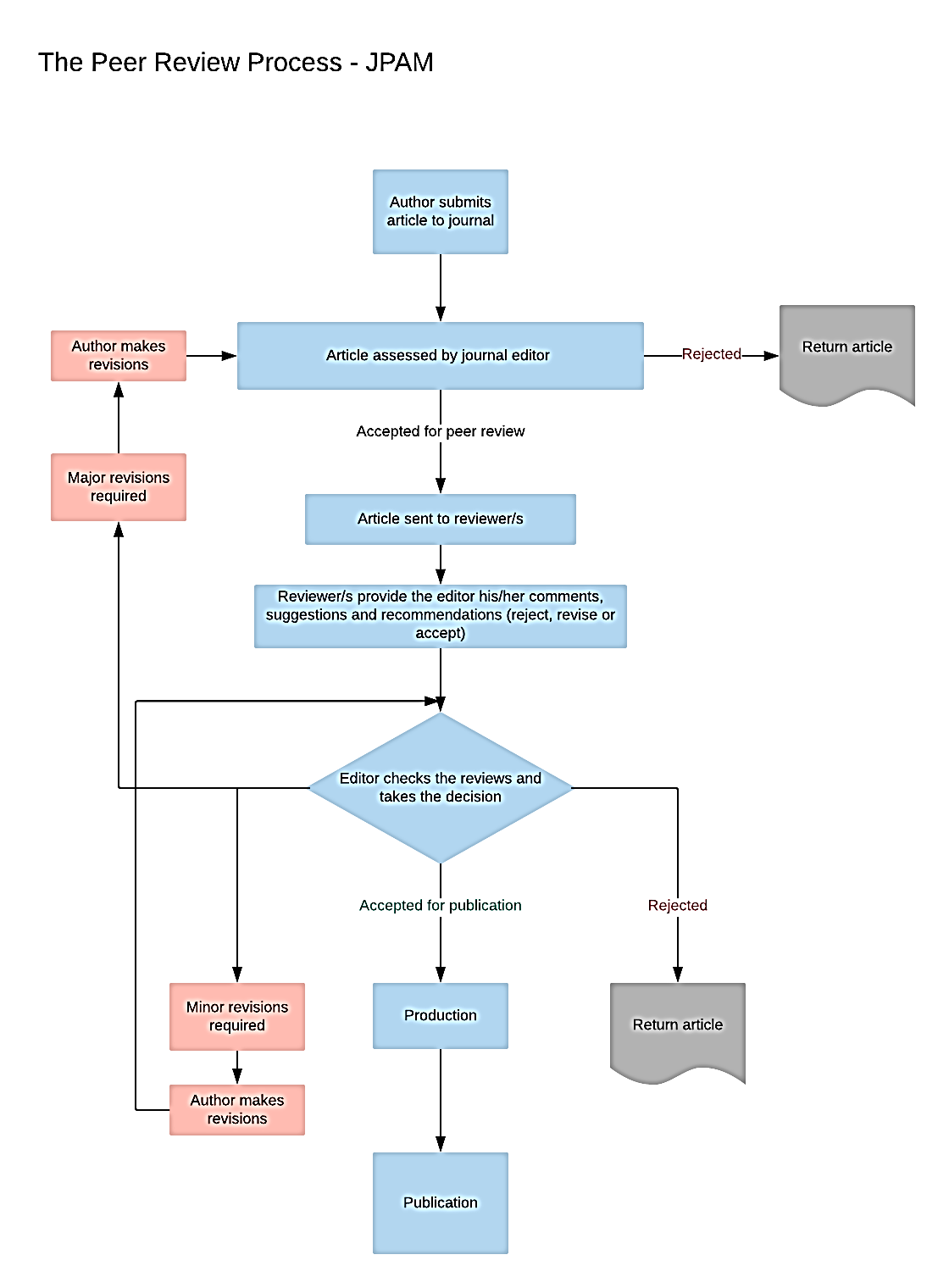
All articles undergo peer review based on the following criteria:
Please ensure that the article does not exceed a length of 2500 words, excluding the Abstract and References sections. Original research articles should include a structured abstract with four subheadings, limited to a maximum of 250 words:
(i) Background & objectives, (ii) Methods, (iii) Results, and (iv) Interpretation & conclusions, followed by 5-8 key words arranged alphabetically. For the main article, it is recommended to arrange the sections in the following order: Introduction, Materials and Methods, Results, Discussion, Acknowledgments (if applicable), Conflicts of Interest, and References. Obtaining permission from the Ethics Committee or Institutional Review Board (IRB) is a crucial requirement for all studies involving human subjects and animals. It is essential to include this information in the Materials & Methods section. Registration of clinical trials is a requirement, and it is essential to include the registration number/CTR number.
The AR Publishers publishes a range of review articles, including rapid reviews, mapping reviews, scoping reviews, and more. Articles written by scientists or experts who have published high-quality, original research in the specific area will be considered. For the article, it would be ideal to have a word count of around 4000 words, excluding the Abstract and References. It's essential to keep the number of references to a maximum of 100, ensuring they are recent and relevant. Additionally, please provide a transparent methodology that outlines the search strategy used. Lastly, include an unstructured abstract of approximately 250 words. Tables and Figures can be included as needed. Before reproducing a published Table/Figure, it is essential to obtain copyright permission from the copyright holder.
The articles in this section will provide a thorough evaluation of various studies on significant clinical and public health topics. The goal is to obtain an impartial quantitative estimate of the overall impact of an intervention or variable on a specific outcome. The emphasis could be on cause, diagnosis, prognosis, therapy, prevention, and other related aspects. These articles would be meticulously researched, providing a comprehensive and unbiased perspective. It would be beneficial to include a structured abstract. Systematic reviews typically have a word limit of 2,500 words and should consist of a limited number of tables and figures. These will undergo a thorough peer review process before being published.
If you have a research manuscript with a well-defined study design and sample size, but limited parameters analysed, you can submit it as a Short Paper. These documents will consist of approximately 2000 words and include a well-organized abstract that combines the Results and Discussion sections. A Research Correspondence can take the form of either a preliminary/pilot study or a post-implicative report, without including an abstract. If you have preliminary investigative data with limited methodology and sample size, but it has important clinical implications, you can submit it as a Research Correspondence. This would consist of approximately 1000-1500 words and may include a Table and/or a Figure.
There is no fee for submitting and processing your manuscript with Journals operated by AR Publishers. For publication in the journals, it is essential to include the following points in all submitted manuscripts: 1. First page 2. Article page 3. Tables & Figures 4. A scanned copy of the ethical clearance certificate 5. Undertaking by authors & copyright transfer agreement. The details are provided below.
Please ensure that the file contains a Covering letter, a Title page, and the Author's contribution.
When submitting your paper, it is essential to provide a clear overview of why it is best suited for publication, as opposed to a specialty journal. It is important to designate one of the authors as the corresponding author for the paper. This individual will be responsible for both the content of the paper and communication with the Editorial office. The author must clearly state that the article has not been published or is currently being considered for publication, in whole or in part, in any other journal or conference proceedings.
The title page should include the names of the author(s), their highest degree, the name(s) of the department(s), the academic positions of the authors in the department, the complete postal addresses, mobile numbers, and email addresses of all authors, and the name of the corresponding author with all the aforementioned details.
The title page should include during manuscript submission: (i) Type of manuscript: original article/ review/ correspondence/ perspective/ view point/ clinical image/ letter to editor; (ii) Title; (iii) Short title; (iv) Number of Tables; (v) Number of Figures; (vi) Source of financial support in the form of grants; (vii) Registration number in case of Clinical Trials.
The author's specific contribution should be acknowledged on the Title page.
Please submit your manuscripts online through the website: https://www.arpublishers.com by following the online submission instructions. It is essential to present manuscripts in a clear and organized manner, with a focus on conciseness and neatness. It is essential to ensure that pages are numbered consecutively and that the contents are arranged in the following order:
Title:
The title of the article should be brief, continuous (no broken or hyphenated titles), and descriptive and informational enough to be effective in indexing and information retrieval
A short running title not exceeding 6-7 words must also be provided. Abstract & Key words:
It is recommended that all manuscripts, excluding review articles, include a structured abstract of approximately 250 words. The abstract should be organized with subheadings for Background and Objectives, Methods, Results, Interpretation, and Conclusions. The abstract should be concise and provide an overview of the paper's scope and essential findings. The focus should be on highlighting the main findings and conclusions, allowing abstracting services to use it as is. It is essential to exclude findings and recommendations from the Abstract that are not substantiated by the article's text.
A set of suitable keywords (4-8 in number) arranged in alphabetical order should also be provided.
Introduction:
The introduction should be concise and clearly outline the paper's scope. Reviewing the literature should focus solely on the reasons for conducting the current study and provide only the most crucial background information. It is essential to ensure that the objective of the study is clearly stated and justified at the end of this section.
Material & Methods:
It is essential to include the nomenclature, source of materials and equipment used, along with the manufacturers' details in parentheses. It is essential to clearly state the procedures so that other workers can easily replicate the results, if needed. It is necessary to provide detailed descriptions of new methods, including any potential limitations they may have. When discussing established procedures, it is essential to offer authentic references and, if applicable, explain any significant deviations and the reasons behind their adoption. When reporting experiments on human subjects and animals, it is essential to clearly state that the procedures followed adhere to the ethical standards set by the national bodies or organizations of the respective country. When conducting research in India involving human subjects, it is essential to adhere to the ICMR's Ethical Guidelines for Biomedical Research on Human Participants (2017). When conducting experiments on laboratory animals, it is essential to adhere to the guidelines established by reputable organizations, such as the ICMR, INSA, or CPCSEA. These guidelines provide valuable instructions on the proper care and use of animals in scientific research. It is essential to provide sufficient information regarding the care and use of laboratory animals, including details on their source, strain, age, sex, housing, and nutrition, among other factors. It is essential to ensure that the necessary certification is included when submitting manuscripts. It is essential to provide accurate and detailed information about the drugs and chemicals used, including their generic names, dosages, and routes of administration.
Study Design:
It is essential to clearly describe the selection process for observational or experimental participants, including patients or laboratory animals, as well as the controls. This should also include information about whether the selection was random or consecutive. Additionally, the basis for calculating the sample size should be provided, along with the eligibility and exclusion criteria, as well as a description of the source population.
The period (including month and year) and location of the study should be clearly stated.
Contributors may consult the following Guidelines for specific study designs:
| Sr. No. | Type of study | Source |
|---|---|---|
| 1 | Randomized controlled trials (RCTs) | CONSORT- http://www.consort-statement.org |
| 2 | Systematic reviews & meta-analysis | PRISMA guidelines - http://www.prisma-statement.org |
| 3 | Observational studies in epidemiology | STROBE - http://www.strobe-statement.org/ |
| 4 | Meta-analysis of observational studies in epidemiology | MOOSE - http://statswrite.eu/pdf/MOOSE%20Statement.pdf |
| 5 | Studies on diagnostic accuracy | STARD - http://www.stard-statement.org |
Clinical trials study:
It is essential for all clinical trials to be registered in a Primary Clinical Trial Registry and for the Registration number to be provided under Materials & Methods. This ensures the transparency and credibility of the studies based on clinical trials. Articles that present results of randomized clinical trials should include comprehensive information about key study elements. This includes details about the study protocol, such as the methods used for assigning interventions (including randomization and concealment of treatment group allocation) and the method of masking (also known as blinding). The CONSORT Statement (http://www.consort-statement.org/) provides guidelines for reporting these elements. It is essential to note that the study protocol was approved by the institutional ethics committee, and written consent was obtained from all participants.
It is important to mention the statistical analysis conducted and the statistical significance of the findings, when applicable. Unless it is essential for a clear understanding of the article, it is best to avoid providing a detailed description of the statistical treatment. When it comes to articles that heavily rely on statistical considerations, it is essential to provide detailed information, particularly when employing new or uncommon methods. Authors should provide authentic references for standard and routine statistical methods used.
Results:
It is important to ensure that the data is organized consistently and logically to enhance the clarity and coherence of the report. It is important to avoid duplicating data from Tables and Figures in the text. It is crucial to highlight or condense only the significant observations. The same data should not be presented in both tabular and graphic forms. It is important to note that the interpretation of the data should be reserved for the Discussion section, rather than being included in the Results section.
Discussion:
The discussion should focus on interpreting the results, avoiding repetition of information already covered in the Results section. It is important to establish connections between new findings and existing knowledge, while also incorporating logical deductions. It is important to address any weaknesses, limitations, or gaps in the study.
The conclusions align with the study's goals. However, it is crucial to avoid making unqualified statements or drawing conclusions that are not fully supported by the data. Avoid claiming priority on ongoing work. It is essential to clearly identify any warranted hypotheses. Recommendations should only be included in the Discussion if they are absolutely necessary and relevant. It would be ideal to conclude this section with a final remark.
Acknowledgement:
Acknowledgement should be concise and limited to particular scientific/technical support rather than normal departmental facilities and encouragement or aid with paper preparation (including typing or secretarial assistance).
Financial support & Sponsorship:
Funding support and/or sponsorship received from national or international funding agencies should be acknowledged.
Conflict of Interest:
There is a conflict of interest when authors or their institutions have financial or personal relationships with other individuals or organizations that could improperly influence (bias) their actions. A conflict of interest may be actual or potential, and complete disclosure to the editor is required. All submissions must disclose all relationships that could be construed as posing a conflict of interest.
All authors are required to disclose any financial and personal relationships with other individuals or organizations that could improperly influence their work (bias). If no conflicts of interest exist, authors should state so.
Referencing:
It is essential to cite the reference in accordance with the APA reference style. Please make sure that each and every reference cited in the text must also be present in the Reference list and vice versa. A manuscript that does not follow the reference citation rule of journals will be returned to the author before undergoing any review or initial screening.
The references in the text must be written as
(i) If the article has a Single Author, then (Last name of First Author, year of publication): for example (Purple, 2023)
(ii) If the article has Two Authors, then(Last name of First Author and Last name of Second Author, year of publication): for example (Purple and Black, 2023)
(iii) If the article has three or more Authors, then(Last name of First Author et al., year of publication): for example (Purple et al., 2023)
(iv) If the information or data is extracted from more than one reference, then the references must be arranged chronologically and separated by a semicolon. For example (Purple et al., 2020; Black B et al., 2021; Red & White, 2023)
The Reference List is provided as follows:
Arrange the entries in Alphabetical order as mentioned in the table with particular attention to sequence, punctuation, spacing, and capitalization.
| Type of Article / Reference | Punctuations and order of elements in the reference list |
|---|---|
| Journal article with 1 author |
Author Name. (Year of Publication). Title. Journal Name, Volume (Issue), Page Number. DOI For Example, |
| Journal article with 2 authors |
Journal article with 2 authors For Example, |
| Journal article with 1-3 authors |
Authors Name. (Year of Publication). Title. Journal Name, Volume (Issue), Page Number. DOI For Example, |
| Article with same authors in the same order |
Arrange by year of publication. For Example, |
| Edited Book |
Editor/s Name (Eds.). (Year of Publication). Book title. Journal/ Publisher Name. DOI For Example, |
| Chapter in an Edited Book |
Authors Name. (Year of Publication). Chapter Title. In Editor/s Name (Eds.), Book title (Page No.). Journal/ Publisher Name Name. DOI For Example, |
| Authored Book |
Authors Name. (Year of Publication). Book title.Journal/ Publisher Name. DOI For Example, |
| Conference Session |
Conference Session Authors Name. (Year of Publication, Month Date). Title [Conference session]. Theme of the Conference, Location, Country. Web Address. |
| Webpage |
Authors Name. (Year of Publication, Month Date). Title. Web Address |
Tables must be consecutively numbered using Roman numerals (I, II, III, etc.). They should have concise titles, and column headings should be brief as well. The abbreviated units of measurement should be written beneath the headings. Variations in statistical measurements, such as standard deviation (SD) and standard error (SE), should be identified. Avoid the inclusion of structural formulas in Tables. Include abbreviations in the footnote.
Illustrations should be submitted in JPEG or TIFF format (with a file size of no more than 1 MB), numbered consecutively in Arabic numerals, and accompanied by an appropriate Title and explication of symbols in the inscriptions. In the upper left corner of a multi-panel diagram, the various portions should be labeled A, B, C, and so on.
Photomicrographs should include internal scale markers to facilitate accurate magnification details for final print reduction. In photomicrographs, symbols, arrows, and letters should be legible and contrasted against the background. As Figures, JPEG/TIFF-formatted graphs can be uploaded.
All published materials must be acknowledged, and copyrighted materials must be submitted with the copyright holder's written permission.
Only standard abbreviations are permitted. Throughout the text, tables, and figures, abbreviations should adhere to the International System of Units (SI) standards. The drugs' generic names should be used. If proprietary trademarks are used in research, the brand name, manufacturer, and country should be included in parentheses following the generic name at the first occurrence
All studies involving patients, volunteers, human biological material, or animals must submit a scanned copy of their Ethical Clearance Certificate.
APC prices are set on a per journal basis to accommodate the diverse scopes, disciplinary areas, and publishing requirements of each journal.
| Journal Title | Article Processing Charge | |
|---|---|---|
| High Income Countries | Lower Middle Income Countries | |
| International Journal of Multidisciplinary Health Sciences and Research | 150 USD | 85 USD |
| Global Journal of Multidisciplinary Health Research (GJMHR) | 150 USD | 85 USD |
Stay ahead in the ever-evolving digital landscape with AR Publishers. Join our community to access exclusive insights tools.